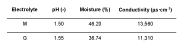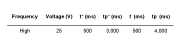The present work focuses on evaluating the roughness evolution by using the DryLyte® Technology on CoCr-Removable Partial Dentures (RPD) to reduce the initial roughness and be able to produce a smooth area by using a macroporous and gel electrolyte. The quality of the surface has been evaluated by using advanced characterization techniques. To sum up, both electrolytes present a bi-modal particle size distribution, and the macroporous one allows to obtain a mirror-like surface CoCr-RPD specimen.
1. Introduction
Removable partial dentures (RPD) are removable prosthetics made for the dentistry sector. The main goal of RPDs is to provide prosthetic rehabilitation of missing teeth and associated structures, avoiding further loss of remaining teeth. RPDs present four major metal components: connectors, rests, clasps, and mesh bases. Except for clasps, each component should be rigid and resist permanent deformation, which suggest a material of high elastic modulus and yield strength [1]. Currently, CoCr alloys are applied in prosthodontics due to their excellent biocompatibility, high-corrosion resistance, relative affordability, and good mechanical properties [2-5]. Traditionally, the CoCr-RPD were made using the lost wax-casting method, which is heavily dependent on technical ability, and many errors are related to human capacity. Also, if molten alloy is overheated, the final component will present internal cracks due to the thermal expansion and on the other hand, the temperature is not high enough, and the alloy may not fulfill the mold [3-6]. Nowadays, computer assisted designing (CAD), computer assisted manufacturing (CAM) as well as casting and additive manufacturing are used to design and/or fabricate the RPD. Both techniques shorten production time, reduce the human error, and increase the productivity of high-quality of CoCr-RPD specimens.
Once the CoCr-RPD specimen is manufactured, it is occasionally necessary to carry out a post-processing operation to get the desired specifications in terms of superficial roughness. In this sense, grinding and subsequently polishing process needs to be done to achieve a mirror-like surface. In this regard, the main requirement of doing a post-processing technique on CoCr-RPD specimens is to preserve initial shape and respect tolerances, as the framework needs to perfectly fit patient mouth. In addition, surfaces have to achieve a very low roughness, be easy to clean and corrosion-resistant, and have no pores where bacteria can get trapped.
Within the aforementioned information, the main goal behind this research is to use a new dry electropolishing technology to achieve the requirements of CoCr-RPD by means of the DryLyte Technology. Two different commercial resins (labeled as macroporous “M” and gel “G”) are used to compare the effectiveness of both electrolytes on CoCr-RPD.
2. Experimental procedure
A commercial CoCr-RPD specimen was polished by using the DryLyte® Technology for 25 min by using a macroporous (labelled as “M”) and gel (labelled as “G”) resins. The main physical properties (pH, moisture, and conductivity) as well as the electrical parameters employed to electropolish the CoCr-RPD specimen are summarized in Tables 1 and 2, respectively. A non-polished specimen (labelled as as-received material, AR) has been also used as a reference in terms of roughness.
The particle size as well as the microstructural parameters were evaluated by using advanced characterization techniques. To evaluate the particle size distribution of both electrolytes, twenty images per electrolyte were taken by using Neox S Confocal (Sensofar Metrology), and the diameters measured by using the ImageJ software by directly measuring at least 600 electrolyte particles. Subsequently, the particle size distribution was treated by using a statistical methodology proposed by Ulm and Constantinides [7-9]. The infrared spectra were obtained by using an IR Nicolet 6700 Fourier Transform InfraRed (FT-IR) spectrophotometer from Thermo Scientific equipped with Csl beamsplitter. The FT-IR spectra for the investigated electrolytes were recorded in the 4,000 – 500 cm-1 range, with a 4 cm-1 resolution (128 scans collected).
The roughness evolution and 3D profile were measured by means of Neox S Confocal (Sensofar Metrology). All measurements were taken with Nikon-EPI 10x, the area investigated was 4.23 mm x 3.54 mm, and the cut-off used was 2.5 mm. All the roughness parameters were calculated according to the ISO 4287.
3. Results and discussion
3.1. Particle size distribution
The mean electrolyte particle size and distribution were measured for the different electrolytes investigated here (M and G) by direct measurement of the electrolyte diameter. Figure 1 displays the histogram of the measured electrolyte particle size diameter with a constant bin size of 50 μm, obtained from an average of at least 600 spheres for both electrolytes. The insert inside the histogram shows a visual image of the employed electrolyte. As depicted in this figure, a bimodal particle size distribution was obtained. As depicted in this representation, the first and second peak correspond to a fine and coarse particle, respectively. Table 3 summarizes the particle size for both investigated electrolytes.
3.2. Electrolyte composition
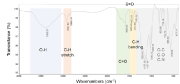
The FT-IR spectrum of M electrolyte is shown in Figure 2. The spectrum for the G electrolyte is not shown here since it is similar to the one presented for the M electrolyte. Furthermore, three different regions are clearly visible in the spectra, in agreement with the data reported in [10]. In this sense, each region corresponds to (1) Region 1: high wave numbers, ranging from 3,750 up to 3,000 cm-1 which corresponds to the stretching vibration of the hydroxyl group peak present in the electrolyte; (2) Region 2: intermediate zone, nearing region 1 and presenting an interval below 3,000 cm-1 of wavelength. In this zone bands related with the C-H stretch of the CH2 appear (2,852 and 2,924 cm-1) and (3) Region 3: the lower region which ranges between 1,750 and 500 cm-1. In this region, a high number of different peaks appears, which are attributed to C=O, S=O, C–H bending, C-C, C-O and C-N. Those peaks are mainly related to the investigated electrolyte, styrene divinyl benzene.
3.3. Surface quality
Figure 3 shows a visual aspect of the CoCr-RPDs before and after being dry-electropolished by using the M- and two steps dry-electropolishing process, and by combining a first step with M-electrolyte and subsequently a G-electrolytes to improve the shinning effect. The workpiece electropolished initially with the M-electrolyte and subsequently with the G-electrolyte shows a mirror-like surface while the one polished with M-electrolyte does not shine. The M-electrolyte leads to rapid removal of the macroroughness, without increasing significantly the brightness of the desired specimen. However, in order to achieve the desired specifications in terms of roughness and brightness, it is necessary to conduct the dry-electropolishing process in two steps. By combining the M- and G-electrolyte, it can reduce roughness and achieve an outstanding surface brightness as depicted in Figure 3.
4. Conclusions
From this research, the following conclusions may be drawn:
(1) Both electrolytes present a bi-modal particle size distribution with a spherical geometry. The particle size for the M- and G-electrolytes ranges between 406 and 628 μm, respectively.
(2) The FT-IR spectrum presents the main electrolyte constituents, being mainly the water (H-O), styrene divinyl benzene groups, etc.
(3) The CoCr-RPDs can be successfully electropolished by using two dry-electropolishing steps, initially by using the M-electrolyte to rapidly reduce the macroroughness and finally a second step by using the G-electrolyte to achieve the desired superficial roughness and brightness.
References
[1] M. B. Fathi, B. Rezai, E. K. Alamdari, R. D. Alorro. Mechanism and equilibrium modeling of Re and Mo adsorption on a gel type strong base anion resin. Russian Journal of Applied Chemistry. 90 (2017) 1504.
[2] H. Wang, J. B. Xu, Z. Nie, W. Y. Ma, Q. M. Zhang, L. Guo. Preparation and properties of Co-Cr alloy denture by selective laser melting. Materials Research Express. 6 (2018) 026552.
[3] B. Galateanu, F. Golgovici, A. Hudita, M. Stan, S. Dinescu, M. Costache, I. Demetrescu, A. Popescu. About electrochemical stability and biocompatibility of two types of CoCr commercial dental alloys. Materials and Corrosion. 67 (2016) 1096.
[4] C. Herpel, A. Springer, G. Puschkin, L. Zimmermann, T. Stober, P. Rammelsberg, F. S. Schwindling. Removable partial dentures retained by hybrid CAD/CAM cobalt-chrome double crowns: 1-year results from a prospective clinical study. Journal of Dentistry. 115 (2021) 103847.
[5] O. Alageel, M. Abdallah, A. Alsheghri, J. Song, E. Caron, F. Tamimi. Removable partial denture alloys processed by laser-sintering technique. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 106 (2017) 1174.
[6] O. Barraclough, D. Gray, Z. Ali, B. Nattress. Modern partial dentures – part 1: novel manufacturing techniques. British Dental Journal. 230 (2021) 651.
[7] F. Ulm, M. Vandamme, C. Bobko, J. A. Ortega. Statistical Indentation Techniques for Hydrated Nanocomposites: Concrete, Bone, and Shale. Journal of the American Ceramic Society. 90 (2007) 2677.
[8] G. Constantinides, F. Ulm, K. Van Vliet. On the use of nanoindentation for cementitious materials. Materials and Structures. 36 (2003) 191.
[9] G. Constantinides, K. S. Ravi Chandran, F. J. Ulm, K. J. Van Vliet. Grid indentation analysis of composite microstructure and mechanics: Principles and validation. Materials Science and Engineering A. 430 (2006) 189.
[10] M. Fathy, Th. Abdel Moghny, A. ElsayedAwad Allah, A. -H. A. -A. El-Belihi. Preparation of Anion-Exchange Resin from Styrene-Divinyilbenzene Copolymer with High Cross-Linking Structure. Applied Chemistry. 60C (2013) 16098.
For Information:
Steros GPA Innovative, S.L., C/ Maracaibo, 1. 08030 Barcelona, Spain
Tel. +34.931.256 536
E-mail: jj.roa@gpainnova.com