E-Archive
Articles
in Vol. 14 - September Issue - Year 2013
Effect Of Surface Nanostructuration On Tribocorrosion Resistance
![Figure 1: schematic of mechanisms to reduce grain size, according to [1]](https://mfn.li/storage/e-archives/article-pictures/1286/3571.gif)
Figure 1: schematic of mechanisms to reduce grain size, according to [1]

Figure 2: tribocorrosion test device (Ecole Centrale Paris)
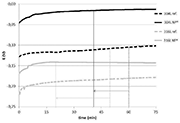
Figure 3: free potential against time in the absence of friction (Phase 1)

Table 1: results of the tests without friction (phase 1)
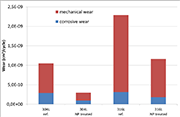
Figure 4: mechanical and corrosive wear under continuous friction (phase 2)
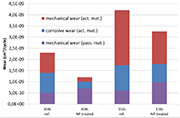
Figure 5: Mechanical and corrosive wear under intermittent friction (phase 3)
Introduction
Recent years have seen the development of many techniques to reduce the grain size at the surface of metallic materials. These methods are based on the principle of Severe Plastic Deformation (SPD) where the initiation of dislocations results in the apparition of grain boundaries within the original grains, leading to the refinement of the microstructure as illustrated by the diagram of Figure 1.
These treatments include SMAT (Surface Mechanical Attrition Treatment), HPT (High Pressure Torsion) or ECAP (Equal Channel Angular Pressing, [1]) or else NanoPeening® developed and patented by Winoa. As it is derived from blasting, the latter presents the advantage of being compatible with industrial applications. This solution allows for the obtention of a top layer, 50 to about 150£gm in thickness, characterized by a gradient of grain size (50-100nm at the surface).
Surface nanostructuring enhances some of the material properties, in particular the resistance to abrasive wear due to a high level of hardness. But in many applications, materials are not only subject to mechanical wear but also to various aggressive conditions such as corrosive environments. The combination of these various constraints creates an effect of "synergy" and the degradation of the material may be accelerated. This is the case, for instance, in tribo-corrosion, where a surface is subjected to friction within a corrosive environment. It was discovered that surface nanostructuring greatly improves the resistance of materials to such a combination of stresses. This study was carried out in collaboration with the team of Professor Pierre Ponthiaux from LGPM laboratory (Ecole Centrale Paris).
Experimental: tribo-corrosion tests
The tests were carried out with a device based on a tribometer where the working head, in sintered alumina, has a circular movement, which imposes a more constant sliding rate than in the case of linear travel. The set-up is shown on Figure 2.
The device has been adapted to operate in an aqueous medium so that it can be associated with an electrochemical cell to realize potential measurements and impedance spectroscopy in situ. The purpose of the whole test is to distinguish and quantify the mechanical components (due to friction) and chemical (corrosion) of the total wear. In addition, the contribution of the base metal must be isolated from that of the passive film that forms progressively. The total wear can be written as:
Wtot = Wact + Wpass = Wmact + Wcact + Wmpass +Wcpass (1)
Where Wmact and Wcact respectively refer to the mechanical and corrosive wear of the active material while Wmpass and Wcpass relate to the components of the passive film. The total wear Wtot is measured ex situ at the end of the test through an optical analysis of the trace by microtopography. To determine the different components of the total wear, the test procedure includes 3 phases.
Phase 1: pure corrosion test (without friction)
During this phase, the sliding head is not activated. It is a purely electrochemical measurement to check, from a qualitative point of view, the passivation behaviour of the material. Quantitatively speaking, the evolution of free potential over time is registered to determine the characteristic time of passivation (representative of how quickly the protective film forms). Then impedance spectroscopy is used to calculate the passivation current intensity, indicative of how protective the passive film is against corrosion.
Phase 2: corrosion test under constant friction
The sliding head is set in motion without downtime so that the passive film does not have the time to form between each pass at a given point. The whole wear therefore can be attributed to the active material and (1) reduces to:
Wtot = Wact = Wmact + Wcact
Wcact is calculated from the passivation current density determined by impedance spectroscopy like in the first phase.
Phase 3: Testing of corrosion intermittent friction
During this step, the sliding head stops for a while after each round. The duration of the downtime is defined according to the characteristic time calculated in phase 1 to allow for the partial reconstruction of the passive film. Thus, all the terms of (1) contribute to the global wear. As spectroscopy measurements are not possible in the case of intermittent friction, the corrosive component of the active material¡¦s wear is extrapolated from the results of phase 2.
The tests were realized on two stainless steel grades: AISI 304L and AISI 316L. The samples used for the tests were cylinders having 25mm in height and 25mm in diameter. For each grade, half of the samples had one flat side treated by NanoPeening® in order to study the effect of surface nanostructuration. As the tribo-corrosion experiments require a very smooth surface state, and since NanoPeening® tends to generate roughness, the samples were then manually polished (less than 10µm removed) prior to the tests.
Results And Discussion
The evolution of free potential over time registered during Phase 1 is shown on Figure 3.
The characteristic time and corrosion current densities determined for the different samples are displayed in Table 1.
These values show that the nanostructured stainless steels passivate faster than the references. The protective properties of the passive film are unchanged as indicated by the passivation current densities, in the same order of magnitude for both NanoPeening® treated and reference samples, for each grade.
The passive film forms by diffusion of chromium from the volume ("bulk") to the surface. Given that chemical diffusion mainly occurs along grain boundaries, the higher density of the latter, resulting from the grain size refinement, promotes the diffusion of chromium, hence the faster generation of the film. A similar phenomenon was observed by Raman Singh et al [3], although in their study the refinement of grains had been obtained quite differently. They also found that diffusion was favored and suggested to reduce chromium contents while retaining the passivation properties of the stainless steel. The work done by Mao et al on a copper alloy also highlighted that a nanostructured state promotes passivation [4].
The wear components determined by continuous friction (phase 2) are shown in the graph of Figure 3. It can be seen that the two contributions - mechanical and corrosive - related to the active material are greatly reduced for the samples in a nanostructured state, and this trend holds for both grades. As a result, the total wear is reduced by 70 and 50% for grades 304L and 316L respectively.
The results of the test carried out under intermittent regime made during Phase 3 are plotted on the graph of Figure 4.
They confirmed that the active material is more resistant against wear (both mechanical and electrochemical) in a nanostructured form than in a conventional state. However, the wear of passive film due to friction (mechanical wear) is slightly higher for the samples having received a NanoPeening® pre-treatment. One hypothesis to explain the higher mechanical wear of the passive layer lies in the way this film is generated: as it forms more quickly, its cohesion may not be insured and degradation can happen, giving rise to abrasive particles and introducing a third body in the system.
Finally, note that no electrochemical component will appear on the graph for the passive film, which makes sense since by nature it is not sensitive to corrosion.
Conclusion
Pure corrosion tests show that the nanostructured samples passivate faster than the references and the film formed is more protective against corrosion. Under continuous friction, the global wear of the active surface of NanoPeening® pre-treated samples is significantly reduced due to a sharp decrease of the mechanical component. The tests with intermittent friction confirm this trend but the passive film is less resistant to mechanical wear in the case of nanostructured surfaces than in standard samples.
These results are linked to the high density of grain boundaries resulting from the nanostructuring of the surface: the chemical diffusion of chromium is favored, increasing the kinetics of formation of the protective oxide film. Its rapid growth may on the other hand be responsible for its greater vulnerability against mechanical wear.
These observations might pave the way for the development of new stainless steel grades with lower Cr content and preserved passivation properties.
References
[1] Semenova I-P., Raab G-I., Saitova L-R., Valiev R-Z., « The effect of equal-channel angular pressing on the structure and mechanical behavior of Ti¡V6Al¡V4V alloy », Materials Science and Engineering, A vol. 387-389 (2004) 805-808.
[2] Tong W-P., Sui M-L., Lu J., Lu K., Tao N-R., Wang Z-B., « An investigation of surface nanocrystallization mechanism in Fe induced by surface mechanical attrition treatment », Acta Materialia, 50 (2002) 4603-4616.
[3] Singh Raman R-K., Gupta R-K., « Oxidation resistance of nanocrystalline vis-à-vis microcrystalline Fe-Cr alloys », Corrosion Science, 51 (2009) 316-321.
[4] Mao X-Y, Li D-Y, Fang F., Tan R-S., Jiang J-Q, « Can severe plastic deformation alone generate a nanocrystalline structure? », Philosophical Magazine Letters, 90 (2010) 349-360.
For Information: Winoa
528 avenue de Savoie
38570 Le Cheylas, France
Tel. +33.4.76 92 91 47
E-mail: constance.morel@winoagroup.com
www.winoagroup.com
www.wabrasives.com



























